
The molecules may also form rings, which themselves can link with other rings ( Figure 2.13 c). The carbon atoms may bond with atoms of other elements, such as nitrogen, oxygen, and phosphorus ( Figure 2.13 b). In this way, long and branching chains of carbon compounds can be made ( Figure 2.13 a). Any of the hydrogen atoms can be replaced with another carbon atom covalently bonded to the first carbon atom. However, structures that are more complex are made using carbon. The simplest carbon molecule is methane (CH4), depicted here. Figure 2.12 Carbon can form four covalent bonds to create an organic molecule. The simplest organic carbon molecule is methane (CH 4), in which four hydrogen atoms bind to a carbon atom. Therefore, it can form four covalent bonds with other atoms or molecules. Carbon BondingĬarbon contains four electrons in its outer shell. It is the bonding properties of carbon atoms that are responsible for its important role. Other elements play important roles in biological molecules, but carbon certainly qualifies as the “foundation” element for molecules in living things. It is often said that life is “carbon-based.” This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many, if not most, of the molecules found uniquely in living things. In addition, they may contain hydrogen, oxygen, nitrogen, phosphorus, sulfur, and additional minor elements. Biological macromolecules are organic, meaning that they contain carbon. Combined, these molecules make up the majority of a cell’s mass. There are four major classes of biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids), and each is an important component of the cell and performs a wide array of functions.

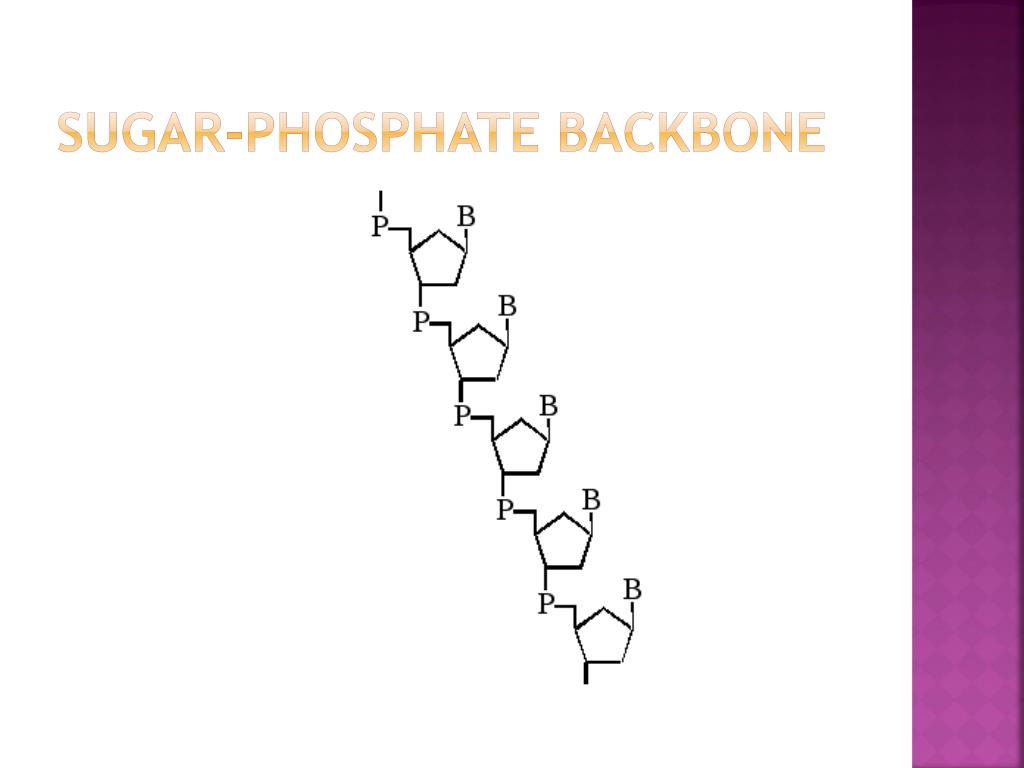
When nucleotides connect to form DNA or RNA, the phosphate of one nucleotide attaches via a phosphodiester bond to the 3-carbon of the sugar of the next nucleotide, forming the sugar-phosphate backbone of the nucleic acid.The large molecules necessary for life that are built from smaller organic molecules are called biological macromolecules.
#HYDROPHILIC SUGAR PHOSPHATE BACKBONE FREE#
A free nucleotide may have one, two, or three phosphate groups attached as a chain to the 5-carbon of the sugar. The number 5 carbon of the sugar is bonded to the phosphate group.
DNA is going to be found in the NUCLEUS of the cell! Online Definition: The base is attached to the primary or first carbon. Nitrogenous bases are covalently bonding with the deoxyribose sugar and phosphate but the bases are ionically bonding together right down the middle. Purine (adenine and guanine) always bonds with the Pyrimidine (thymine and cytosine).
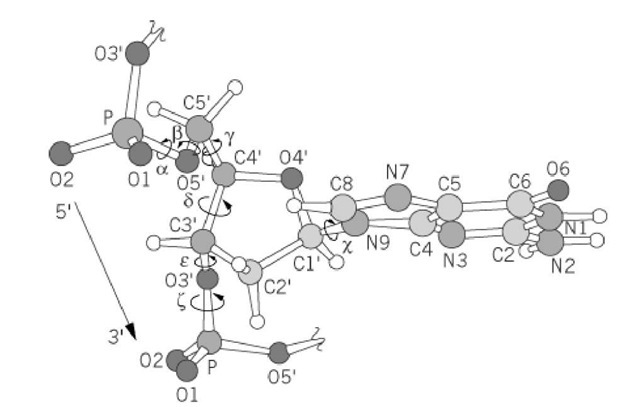
These are going to be held together by a COVALENT BOND! The "rung" of the ladders are going to be the nitrogenous bases that are going to be held together by IONIC (hydrogen) BOND! It's going to be Adenine with Thymine and Guanine with Cytosine. The "backbone" of the DNA are the 2 parallel ladders that are going to be held together by deoxyribose sugar and the phosphate. You're going to want to weld the sugar and the phosphate together as well with all the nitrogenous bases (the A C G T).


 0 kommentar(er)
0 kommentar(er)
